Before Print. Before Launch.
Design is only part of the story. Before print, there’s a regulatory layer that deserves equal attention. Here’s what to know about EU packaging norms.
For founders and designers in beauty, wellness, and lifestyle, EU packaging regulation is often the least visible, yet most decisive, layer of the launch process. It rarely appears in moodboards. It’s not part of brand storytelling. And yet it can determine whether your product reaches the shelf smoothly, or returns from print with costly revisions.
This article isn’t a legal deep dive. It’s a strategic reminder of what every startup should pause and double-check before going to print.
Because compliance is not separate from design.
It’s part of the infrastructure of a serious brand.
The First Misunderstanding: Cosmetics Are Not Food
One of the most common packaging mistakes we see is this:
Applying food labeling rules to cosmetic products.Food and cosmetics are regulated under entirely different EU frameworks. And the details matter. Food law defines strict typographic minimums, including a legally defined minimum x-height for mandatory text. Cosmetic law does not.
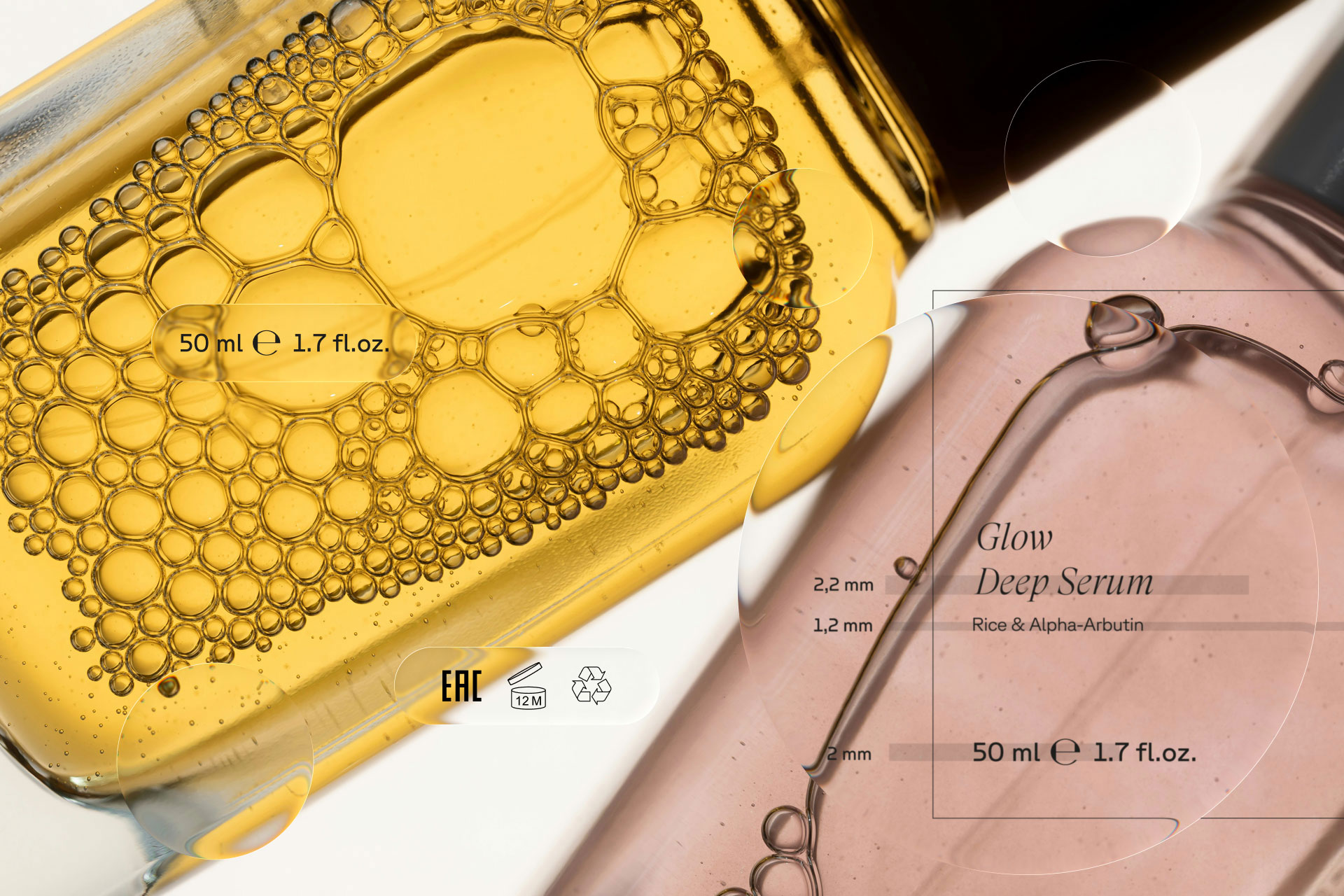
Under the EU Cosmetic Regulation (EC) No 1223/2009, mandatory information must be: Easily legible, Clearly visible, and Indelible.
There is no fixed millimeter requirement for ingredient lists or warnings in cosmetics. Instead, authorities assess readability in context. This distinction alone prevents countless unnecessary layout constraints, or worse, false assumptions. But cosmetics are not unregulated. There is one measurement you absolutely must get right.
The One Fixed Measurement: Nominal Content
Your nominal content (for example, 50 ml) is regulated under EU weights and measures law. Unlike general cosmetic text, this has a defined minimum numeral height depending on fill volume. And here is where precision matters: It refers to the full height of the numerals, not the x-height of your typeface. If this detail is overlooked, your packaging is technically non-compliant, even if everything else is correct. For early-stage brands, this often surfaces too late, after artwork is approved. Before you go to print, this is non-negotiable to verify.
The e-Mark: Strategic, Not Automatic
The ℮-mark (estimated sign) indicates compliance with the EU average quantity system and facilitates free movement of goods across EU member states. But it is not mandatory.
Using it means your filling process must meet specific statistical tolerances and documentation standards. For startups launching locally, the decision is strategic. For brands planning cross-border distribution, it often becomes practical. The key is intentionality. Include it because it aligns with your distribution model, not because “everyone does.”
The Surface Area Exception
If your packaging surface is below 80 cm², certain information (like the ingredients list) may appear on a leaflet or tag, accompanied by the appropriate symbol. However, if your product has an outer box exceeding that threshold, the exception generally no longer applies.This is where many founders misinterpret flexibility. Always assess the full packaging system, not just the bottle.
The Invisible Risks Before Launch
Most compliance issues do not come from dramatic mistakes.
They come from small oversights:
- Ingredient list too small to read comfortably
- Missing local language
- Incorrect responsible person details
- Certification logos downloaded from Google instead of from the issuing body
- Nominal content sized incorrectly
- Claims that unintentionally shift a cosmetic into medical territory
None of these feel dramatic during design. All of them become dramatic at customs, retail onboarding, or market inspection.
The Rules We Always Recommend Before Printing
No matter how small or early-stage your brand is, build this into your process:
1. Always check the current EU regulation — not a blog summary.
Regulations evolve. Enforcement interpretations shift. If you are launching in multiple countries, verify national language requirements.
2. Confirm with your regulatory consultant before final approval.
Design decisions should be aligned before artwork sign-off, not after files are sent to print.
3. Speak with your packaging supplier.
Die-lines, printable areas, varnish zones, embossing, and minimum line weights can impact legibility and compliance. Suppliers often catch issues designers overlook.
4. Only use certification logos provided by the issuing authority.
Whether it’s organic certification, dermatological testing, or sustainability seals — never recreate or approximate logos. Always obtain the official files and usage guidelines directly from the certifying body.
5. Verify your claims language.
Cosmetics cannot make medical claims. Even subtle wording can unintentionally shift regulatory classification.
6. Print a 1:1 proof.
Never judge legibility on screen. What looks refined at 200% zoom can become unreadable at actual size.
7. Clarify the ℮-mark decision early.
This affects both artwork and filling documentation.
Why This Layer Matters for Founders
Compliance isn’t glamorous. It doesn’t live on Instagram. But it’s part of what distinguishes a hobby project from a brand built for scale. A thoughtful brand is not only visually coherent, it is structurally sound.
Retail buyers notice clarity.
Regulators notice precision.
Customers notice ease.
Minimal design does not mean minimal responsibility.
The most elevated brands integrate regulatory information seamlessly — with proportion, hierarchy, and breathing space — without sacrificing aesthetic integrity.
Before You Launch
Pause before you approve the print files.
Ask:
- Is the nominal content correctly sized?
- Are all mandatory elements present?
- Is the language correct for the market of sale?
- Have certifications been applied correctly?
- Has a regulatory professional reviewed the final artwork?
- Has the supplier confirmed technical feasibility?
These are not creative questions.
They are launch questions.
The Full Checklist
If you are preparing to launch, or designing packaging for a client, we created a structured checklist that walks you through everything you need to consider before launching your product. It’s designed for startup founders, beauty entrepreneurs, and designers who want to build brands that are not only beautiful, but ready.
Download now the full EU Packaging Compliance Checklist, or contact us to get help to setup the packaging design files?


.jpg)


